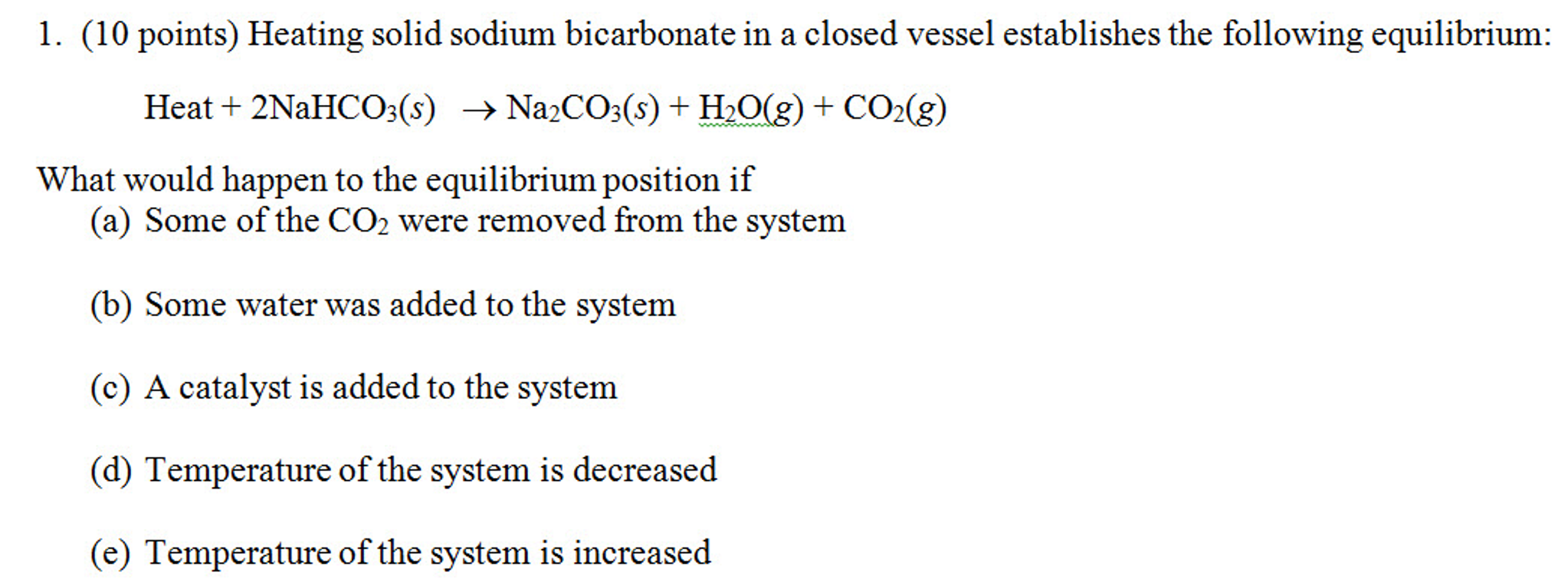
Solved What Would Happen To The Equilibrium Position If Chegg What would happen to the position of equilibrium when the following changes are made to the reaction below? 2hg3o (e)↔6hg (k) o2 (e)Δh2=−25 kj mol (a) hg3o is added to the system (b) the volume of the system decreases (c) temperature increases. your solution’s ready to go!. What would happen if you increased the pressure on this equilibrium? according to le chatelier, the position of equilibrium would move to counter the change. so how does the position shift in this case? the forward reaction produces two molecules of ammonia from a total of four molecules of nitrogen and hydrogen.

Solved What Would Happen To The Position Of The Equilibrium Chegg What would happen to the position of the equilibrium and the value of k when the following changes are made to the equilibrium system below? ch4 (g) 2h2s (g) heat ↔ cs2 (g) 4h2 (g) dh = 21 kj mol. decrease the concentration of dihydrogen sulfide (hydrosulfuric acid). explain your answer. increase the pressure on the system. The position of equilibrium shifts in response to changes such as concentration, temperature, and volume. adding reactants shifts equilibrium forward while removing products shifts it backward. understanding these shifts helps predict the behavior of chemical reactions under different conditions. According to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the fewer number of moles of gas. this particular reaction shows a total of 4 mol of gas as reactants and 2 mol of gas as products, so the reaction shifts toward the products side. What will happen to the chemical equilibrium if hcl is added to the system? the chemical equilibrium will shift to the left. according to le chatelier's principle, what always happens to the equilibrium of a reaction when the temperature is reduced? it shifts in the exothermic direction.

Solved What Would Happen To The Position Of The Equilibrium Chegg According to le chatelier's principle, if pressure is increased, then the equilibrium shifts to the side with the fewer number of moles of gas. this particular reaction shows a total of 4 mol of gas as reactants and 2 mol of gas as products, so the reaction shifts toward the products side. What will happen to the chemical equilibrium if hcl is added to the system? the chemical equilibrium will shift to the left. according to le chatelier's principle, what always happens to the equilibrium of a reaction when the temperature is reduced? it shifts in the exothermic direction. What will happen to the concentration of each reactant and product at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?. Science physics physics questions and answers 4.2 how would the mass \ ( m \) change if the loop came to equilibrium at an angle of \ ( 55^ {\circ} \) ?the mass would stay the samethe mass would decreasethe mass would increaseyou have used 0 of 1 attempt4.3 what would happen to the mass if the length of a side of the square loop was to increase?the mass would increasethe mass. Equilibrium thermodynamics predicts the concentrations (or, more precisely, activities) of various species and phases if a reaction reaches equilibrium. kinetics tells us how fast, or if, the reaction will reach equilibrium. Le chatelier's principle: states that if a system at equilibrium experiences a change in temperature, pressure, or concentration, the system will respond by shifting the equilibrium in a direction to compensate for the change and re establish equilibrium.

Solved What Would Happen To The Position Of The Equilibrium Chegg What will happen to the concentration of each reactant and product at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?. Science physics physics questions and answers 4.2 how would the mass \ ( m \) change if the loop came to equilibrium at an angle of \ ( 55^ {\circ} \) ?the mass would stay the samethe mass would decreasethe mass would increaseyou have used 0 of 1 attempt4.3 what would happen to the mass if the length of a side of the square loop was to increase?the mass would increasethe mass. Equilibrium thermodynamics predicts the concentrations (or, more precisely, activities) of various species and phases if a reaction reaches equilibrium. kinetics tells us how fast, or if, the reaction will reach equilibrium. Le chatelier's principle: states that if a system at equilibrium experiences a change in temperature, pressure, or concentration, the system will respond by shifting the equilibrium in a direction to compensate for the change and re establish equilibrium.

Solved 2 What Would Happen To The Position Of The Chegg Equilibrium thermodynamics predicts the concentrations (or, more precisely, activities) of various species and phases if a reaction reaches equilibrium. kinetics tells us how fast, or if, the reaction will reach equilibrium. Le chatelier's principle: states that if a system at equilibrium experiences a change in temperature, pressure, or concentration, the system will respond by shifting the equilibrium in a direction to compensate for the change and re establish equilibrium.