
Solved Consider The Following Equilibrium For Which Ah Chegg Step 1 considering the given equilibrium equation : 2 so a 2 (g) o a 2 (g) ↽ − − ⇀ 2 so a 3 (g) & given that Δ h <0, this means it is an exothermic reaction a. Equilibrium calculations focus on how reactant and product concentrations change as a system progresses from its initial state to equilibrium. these changes are directly related to the stoichiometry of the reaction. for example, consider the decomposition of ammonia: 2nh3(g) ⇌ n2(g) 3h2(g) 2 nh 3 (g) ⇌ n 2 (g) 3 h 2 (g).

Solved Consider The Following Equilibrium For Which Ah Consider the equilibrium system described by the chemical reaction below, which has a value of kc equal to 7.1 x 10^ 4 at a certain temperature. if a solid sample of cacro3 dissolves in solution, what will the equilibrium concentration of ca2 in the solution be?. What is the answer. consider the following reaction at equilibrium. 2co2 (g) 2co (g) ah = 514 kj 02 (8) le answered step by step ai answer available business • economics question answered asked by chemstemangel what is the answer business economics. Consider the following reaction at equilibrium, for each of the following stresses determine what happen to the reaction (shift product, shift reactant, no shift, cannot be determined).use only the information given here.2 h, 5 (a) 302 (0) h20 (g) 2 s02 (0)1. decreasing the pressure: [ select ]2. adding oxygen: [ select ]3. cooling the the solution: [ select l4. decreasing the size of the. Business economics economics questions and answers question 4consider the following equilibrium: 2h2 (g) x2 (g)⇌2h2x (g) energyaddition of x2 to a system described by the above equilibriumwill cause h2 to decreasewill cause h2x to decreasecannot possibly be carried outwill have ho effectwill cause x2 to decrease.

Solved Consider The Following Equilibrium Chegg Consider the following reaction at equilibrium, for each of the following stresses determine what happen to the reaction (shift product, shift reactant, no shift, cannot be determined).use only the information given here.2 h, 5 (a) 302 (0) h20 (g) 2 s02 (0)1. decreasing the pressure: [ select ]2. adding oxygen: [ select ]3. cooling the the solution: [ select l4. decreasing the size of the. Business economics economics questions and answers question 4consider the following equilibrium: 2h2 (g) x2 (g)⇌2h2x (g) energyaddition of x2 to a system described by the above equilibriumwill cause h2 to decreasewill cause h2x to decreasecannot possibly be carried outwill have ho effectwill cause x2 to decrease. The equilibrium will shift toward reactant but keq will not change, the equilibrium will shift toward product and keq will increase. the equilibrium will shift toward reactant and keq will decrease. Explain your answer consider the following equilibrium cocl 4 2 (aq) 6 h 2 o (l) —> <— co (h 2 o) 6 2 (aq) 4 cl (aq) how would cooling the solution in an ice bath affect its color? explain your answer here’s the best way to solve it. The equilibrium constanth2 (q) i2 (g) 2h (g),kt= [h2]j2 [h2] [u2]0001446consider the following equilibrium reaction and equilibrium constant:2nh3 (g)⇌n2 (g) 3h2 (g)k=9.4is this equilibrium reaction a product favored equilibrium with mostly products, a reactant favored equilibrium with mostly reactants, or an equilibrlum with nearly equal. (a) o₂ (g) is added to the system. the equilibrium will shift toward product and keq will increase. (b) the reaction mixture is heated. the equilibrium will shift toward reactant and keq will decrease. (c) the volume of the reaction vessel is doubled.

Solved Consider The Following Equilibrium Systems Ah 20 0 Chegg The equilibrium will shift toward reactant but keq will not change, the equilibrium will shift toward product and keq will increase. the equilibrium will shift toward reactant and keq will decrease. Explain your answer consider the following equilibrium cocl 4 2 (aq) 6 h 2 o (l) —> <— co (h 2 o) 6 2 (aq) 4 cl (aq) how would cooling the solution in an ice bath affect its color? explain your answer here’s the best way to solve it. The equilibrium constanth2 (q) i2 (g) 2h (g),kt= [h2]j2 [h2] [u2]0001446consider the following equilibrium reaction and equilibrium constant:2nh3 (g)⇌n2 (g) 3h2 (g)k=9.4is this equilibrium reaction a product favored equilibrium with mostly products, a reactant favored equilibrium with mostly reactants, or an equilibrlum with nearly equal. (a) o₂ (g) is added to the system. the equilibrium will shift toward product and keq will increase. (b) the reaction mixture is heated. the equilibrium will shift toward reactant and keq will decrease. (c) the volume of the reaction vessel is doubled.

Solved Consider The Following System At Equilibrium Where Chegg The equilibrium constanth2 (q) i2 (g) 2h (g),kt= [h2]j2 [h2] [u2]0001446consider the following equilibrium reaction and equilibrium constant:2nh3 (g)⇌n2 (g) 3h2 (g)k=9.4is this equilibrium reaction a product favored equilibrium with mostly products, a reactant favored equilibrium with mostly reactants, or an equilibrlum with nearly equal. (a) o₂ (g) is added to the system. the equilibrium will shift toward product and keq will increase. (b) the reaction mixture is heated. the equilibrium will shift toward reactant and keq will decrease. (c) the volume of the reaction vessel is doubled.
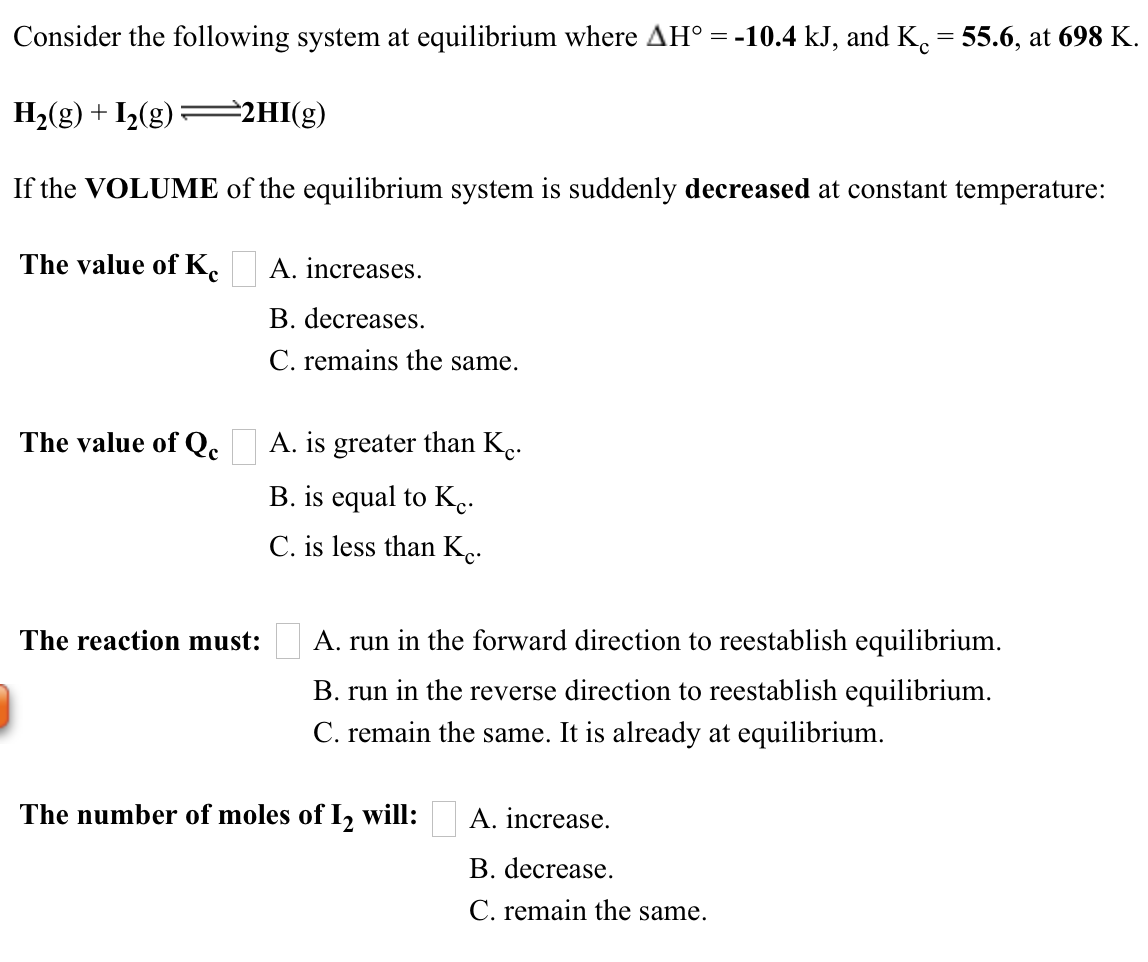
Solved Consider The Following System At Equilibrium Where Chegg