Lab 5 An Equilibrium Constant Part Ii Chegg Once the equilibrium is reached the concentrations of all reactants and products will remain constant since the rate of the forward reaction equals the rate of the reverse reaction. Introduction as mentioned, experiment 34 focuses on determining the equilibrium constant for a chemical system. an equilibrium constant is a value that expresses the relationship between the amounts of products and reactants in a reaction. the amounts are measured in concentration, or molarity.

Solved Module 8 Finding An Equilibrium Constant Post Lab Chegg In this laboratory experiment, a combination of solution chemistry, stoichiometry, and spectrophotometric analysis will be used to determine the equilibrium constant for a reaction between the iron (iii) ion (fe3 ) and the thiocyanate ion (scn ). One of the more important numbers that help us understand an equilibrium system is called the equilibrium constant, keo. for the reaction between fe* and scn, the keg is defined by the equation shown below. Study with quizlet and memorize flashcards containing terms like equilibrium constant for an equation aa bb —> cc dd, k for fe^3 (aq) scn (aq) <—> fescn^2 (aq), beer's law and more. If it did not read zero absorbance, we would have to concentration of the product by the product of the two reactants. the equilibri um constant of solutions 6 10 are all between 77 and 112. the larger the conce ntration of the products, the larger the equilibrium constant. this tell s us that the reaction tends to move.

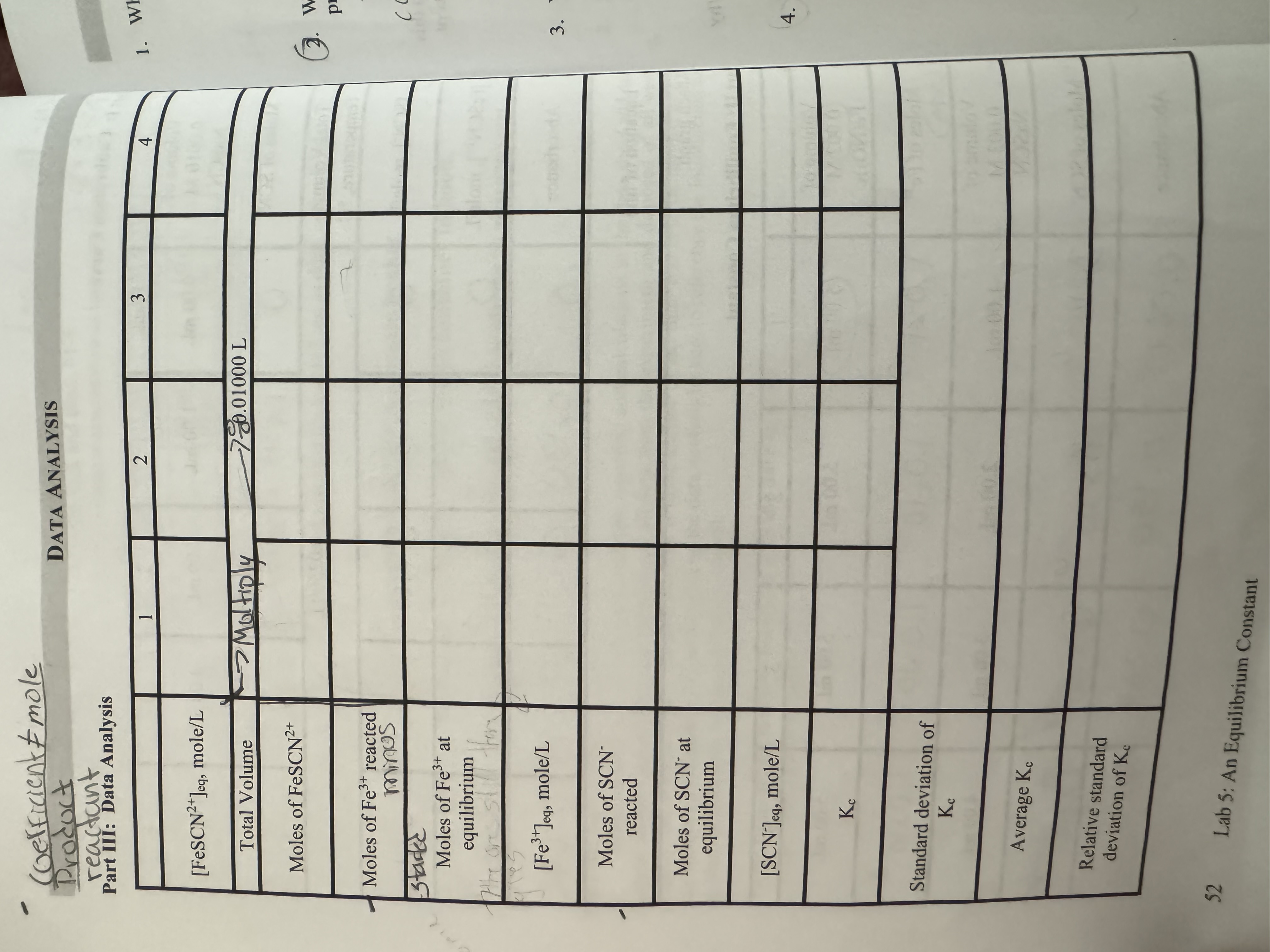
Computer 10 Chemical Equilibrium Finding A Constant Chegg Study with quizlet and memorize flashcards containing terms like equilibrium constant for an equation aa bb —> cc dd, k for fe^3 (aq) scn (aq) <—> fescn^2 (aq), beer's law and more. If it did not read zero absorbance, we would have to concentration of the product by the product of the two reactants. the equilibri um constant of solutions 6 10 are all between 77 and 112. the larger the conce ntration of the products, the larger the equilibrium constant. this tell s us that the reaction tends to move. Answer to equilibrium equilibrium constant (spectrophotometry)equilibrium equilibrium constant (spectrophotometry) introduction laboratory simulation a lab data x solution 3 0.000114 0.144 molar absorptivity (m'cm') 1267 prepared solutions, absorbance, and calculated (fescn24) solution 1 solution 2 solution 3 solution 4 solution 5 0.00200 0.00200 0.00200 0.00200 0.00200 . 0.00200 0.00200 0. The main objective of the lab was to calculate the equilibrium constant keq of the reaction: fe3 scn ⁻⇌fescn2 . the average keq was calculated to be 249 using a variety of techniques to determine equilibrium concentrations of reactants and products such as the ice box. If we measure the concentration of a product, it reaches a constant value short of that predicted by the theoretical yield calculation. in these cases, we say that the reaction has reached equilibrium. we write the chemical reaction using equilibrium arrows instead of a single arrow:. Experiment 5: determination of an equilibrium constant lab report sheet note: many of the calculations can be done prior to coming to lab, based on the concentrations and stoichiometry given in the introduction.