
Solved Which Reaction Essentially Goes To Completion Ok 181 Chegg Question: = understanding that no reaction goes to 100% completion methane and oxygen react to form water and carbon dioxide, like this: ch, (g) 20 (g) 2 h2o (g) co2 (g) use this chemical equation to answer the questions in the table below. This video explains why no chemical reaction goes to 100% completion and how to predict reactant and product amounts at equilibrium.
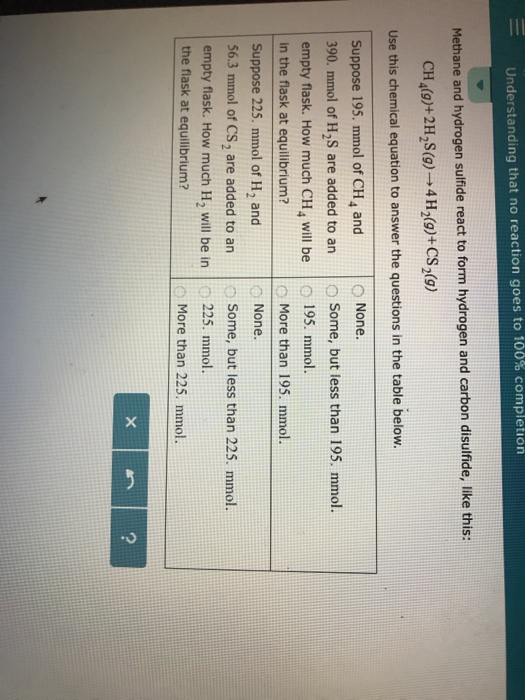
Solved Understanding That No Reaction Goes To 100 Chegg Kinotics and equilibrium understanding that no reaction goes to 100% completion nitrogen dioxide and carbon monoxide react to form nitrogen monoxide and carbon dioxide, like this: no 2 (g) co (g)to no (g) co 2 (g) use this chemical equation to answer the questions in the table below. ×. Solution for understanding that no reaction goes to 100% completion methane and water react to form carbon monoxide and hydrogen like this: ch4 (g) h2o (g) → co (g) 3h2 (g). What is the rate of the reverse reaction just after the n2 and h2o has been added to the flask? greater than zero, and greater than the rate of the forward reaction. = understanding that no reaction goes to 100% completion ammonia and oxygen react to form nitrogen and water, like this: 4 nh, (9) 302 (9) 2n2 (9) 6h,0 (9) use this chemical equation to answer the questions in the table below. suppose 80.0 mmol of nh, and o none. 60.0 mmol of o, are added to an o some, but less than 80.0 mmol. empty flask.

Solved Understanding That No Reaction Goes To 100 Chegg What is the rate of the reverse reaction just after the n2 and h2o has been added to the flask? greater than zero, and greater than the rate of the forward reaction. = understanding that no reaction goes to 100% completion ammonia and oxygen react to form nitrogen and water, like this: 4 nh, (9) 302 (9) 2n2 (9) 6h,0 (9) use this chemical equation to answer the questions in the table below. suppose 80.0 mmol of nh, and o none. 60.0 mmol of o, are added to an o some, but less than 80.0 mmol. empty flask. #### solution by steps ***step 1: identify the given information*** the given chemical equation is: 2c₂h₂ (g) 5o₂ (g) → 4co₂ (g) 2h₂o (g) the first question asks about the amount of c₂h₂ and o₂ added to an empty flask at equilibrium. Question: nderstanding that no reaction goes to 100% completion methan oxygen react to form water and carbon dioxide, like this: ch4 (9) 202 (9) 2h2o (g) co2 (9) use this chemical equation to answer the questions in the table below. Hydrogen and iodine react to form hydrogen iodide, like this:h2 (g) i2 (g) → 2hi (g)use this chemical equation to answer the questions in the table below:#apc. O kinetics and equilibrium 1 5 kevin understanding that no reaction goes to 100% completion v hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this: 2 h, s (g) 30, (9) 2 so, (9) 2h, o (g) use this chemical equation to answer the questions in the table below. suppose 225.
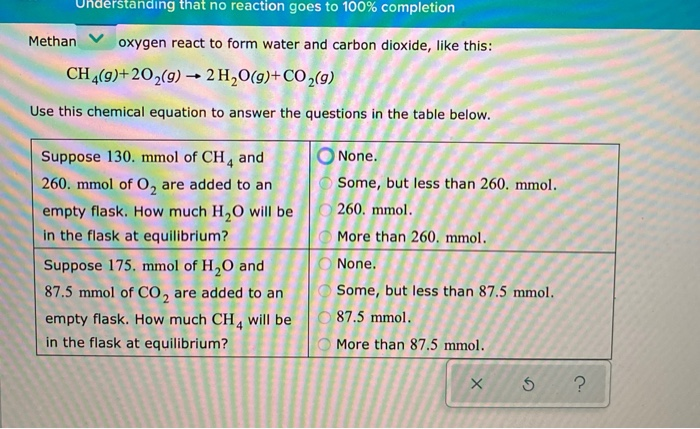
Solved Nderstanding That No Reaction Goes To 100 Completion Chegg #### solution by steps ***step 1: identify the given information*** the given chemical equation is: 2c₂h₂ (g) 5o₂ (g) → 4co₂ (g) 2h₂o (g) the first question asks about the amount of c₂h₂ and o₂ added to an empty flask at equilibrium. Question: nderstanding that no reaction goes to 100% completion methan oxygen react to form water and carbon dioxide, like this: ch4 (9) 202 (9) 2h2o (g) co2 (9) use this chemical equation to answer the questions in the table below. Hydrogen and iodine react to form hydrogen iodide, like this:h2 (g) i2 (g) → 2hi (g)use this chemical equation to answer the questions in the table below:#apc. O kinetics and equilibrium 1 5 kevin understanding that no reaction goes to 100% completion v hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this: 2 h, s (g) 30, (9) 2 so, (9) 2h, o (g) use this chemical equation to answer the questions in the table below. suppose 225.
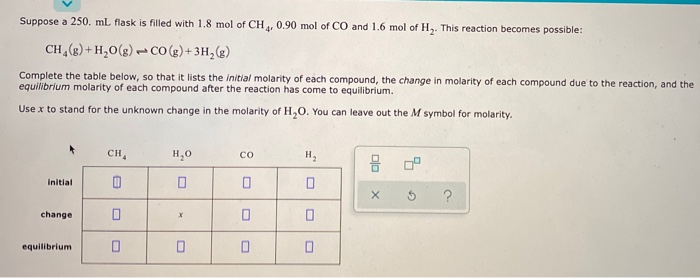
Solved Nderstanding That No Reaction Goes To 100 Completion Chegg Hydrogen and iodine react to form hydrogen iodide, like this:h2 (g) i2 (g) → 2hi (g)use this chemical equation to answer the questions in the table below:#apc. O kinetics and equilibrium 1 5 kevin understanding that no reaction goes to 100% completion v hydrogen sulfide and oxygen react to form sulfur dioxide and water, like this: 2 h, s (g) 30, (9) 2 so, (9) 2h, o (g) use this chemical equation to answer the questions in the table below. suppose 225.