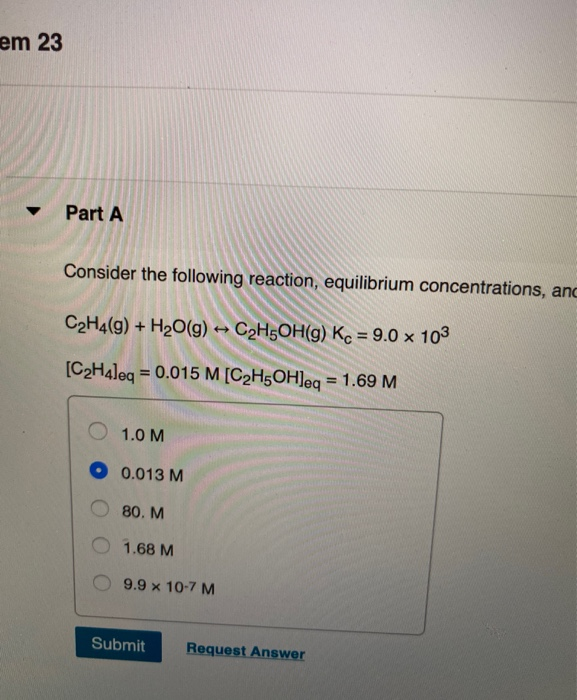
Solved Part A Consider The Following Reaction Equilibrium Chegg Enhanced with ai, our expert help has broken down your problem into an easy to learn solution you can count on. how does the value of k for this new reaction compare to the original reaction, and what is the value of k ' p? here’s the best way to solve it. the equilibrium constant for a reaction is related not the question you’re looking for?. Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particular temperature. determine the equilibrium concentration of so3 (g)and kp in 25ºc. what is the equilibrium constant for the following reaction?.

Solved Part A Consider The Following Reaction At Chegg Consider the following reversible reaction, which is believed to proceed by a one step mechanism in each direction: a. write the rate expression for each direction. b. at equilibrium, the net rate of the reaction is zero, because the rate of the forward reaction is exactly equal to that of the reverse reaction. What is the answer. consider the following reaction at equilibrium. 2co2 (g) 2co (g) ah = 514 kj 02 (8) le answered step by step ai answer available business • economics question answered asked by chemstemangel what is the answer business economics. It follows the stoichiometry of the reaction, making it easier to set up and solve equilibrium expressions. note: an ice table is not a special formula but rather a structured way to apply the fundamental definition of equilibrium—a state in which the rates of the forward and reverse reactions are equal, and concentrations no longer change. The ratio of [no2]2 [n2o4] is not changing. for the reaction 2 so2 (g) o2 (g) ⇌ 2 so3 (g) which of the following is the correct equilibrium constant expression?.

Solved Consider The Following Reaction Equilibrium Chegg It follows the stoichiometry of the reaction, making it easier to set up and solve equilibrium expressions. note: an ice table is not a special formula but rather a structured way to apply the fundamental definition of equilibrium—a state in which the rates of the forward and reverse reactions are equal, and concentrations no longer change. The ratio of [no2]2 [n2o4] is not changing. for the reaction 2 so2 (g) o2 (g) ⇌ 2 so3 (g) which of the following is the correct equilibrium constant expression?. Step 1 c a 2 h a 4 (g) h a 2 o (g) ↽ − − ⇀ c a 2 h a 5 oh (g) we know that the equilibrium constant for reaction is the ratio of conc of products to reactants whe. Consider the following reaction at equilibrium. what effect will removing no2 have on the system? so2 (g) no2 (g) ⇌ so3 (g) no (g) the reaction will shift in the direction of reactants. determine the ka for ch3nh3⁺ at 25°c. the kb for ch3nh2 is 4.4 × 10 4. Write each expression for k k, incorporating all constants, and kp k p for the following equilibrium reactions. given: balanced equilibrium equations. asked for: expressions for k k and kp k p. strategy:. Consider the following reaction at equilibrium, for each of the following stresses determine what happen to the reaction (shift product, shift reactant, no shift, cannot be determined).use only the information given here.2 h, 5 (a) 302 (0) h20 (g) 2 s02 (0)1. decreasing the pressure: [ select ]2. adding oxygen: [ select ]3. cooling the the solution: [ select l4. decreasing the size of the.

Solved Consider The Following Reaction Equilibrium Chegg Step 1 c a 2 h a 4 (g) h a 2 o (g) ↽ − − ⇀ c a 2 h a 5 oh (g) we know that the equilibrium constant for reaction is the ratio of conc of products to reactants whe. Consider the following reaction at equilibrium. what effect will removing no2 have on the system? so2 (g) no2 (g) ⇌ so3 (g) no (g) the reaction will shift in the direction of reactants. determine the ka for ch3nh3⁺ at 25°c. the kb for ch3nh2 is 4.4 × 10 4. Write each expression for k k, incorporating all constants, and kp k p for the following equilibrium reactions. given: balanced equilibrium equations. asked for: expressions for k k and kp k p. strategy:. Consider the following reaction at equilibrium, for each of the following stresses determine what happen to the reaction (shift product, shift reactant, no shift, cannot be determined).use only the information given here.2 h, 5 (a) 302 (0) h20 (g) 2 s02 (0)1. decreasing the pressure: [ select ]2. adding oxygen: [ select ]3. cooling the the solution: [ select l4. decreasing the size of the.

Solved Consider The Following Reaction Equilibrium Chegg Write each expression for k k, incorporating all constants, and kp k p for the following equilibrium reactions. given: balanced equilibrium equations. asked for: expressions for k k and kp k p. strategy:. Consider the following reaction at equilibrium, for each of the following stresses determine what happen to the reaction (shift product, shift reactant, no shift, cannot be determined).use only the information given here.2 h, 5 (a) 302 (0) h20 (g) 2 s02 (0)1. decreasing the pressure: [ select ]2. adding oxygen: [ select ]3. cooling the the solution: [ select l4. decreasing the size of the.

Solved Consider The Following Reaction Equilibrium Chegg