
Solved Practice Equilibrium Problems Calculating K 1 For Chegg Question: practice equilibrium problems: calculating k (1) for the equilibrium reaction below: nh hs (5) nh3 (g) hz5 (g) (a) write the expression for the equilibrium constant for this reaction, in terms of the concentrations, (nh3] and [hzs). Concept question: given a k value of 0.43 for the following aqueous equilibrium, suppose sample z is placed into water such that it’s original concentration is 0.033 m. assume there was zero initial concentration of either a(aq) or b(aq).

Solved Practice Equilibrium Problems 1 Suppose That Each Chegg For the first type of equilibrium problem, we can to solve for k by directly substituting given equilibrium quantities into the reaction quotient: for example, let’s use the following reaction: n2(g) 3h2(g) 2nh3(g). Explain what each of the following values for k tells you about the relative concentrations of the reactants versus the products in a given equilibrium reaction: k = 0.892; k = 3.25 × 108; k = 5.26 × 10 − 11. Chemistry equilibrium practice problems covering equilibrium expressions, concentrations, and le chatelier's principle. We'll learn how to calculate equilibrium concentrations or the equilibrium constant (k) itself using stoichiometry and algebra; specifically, we'll practice how to set up an ice (initial,.
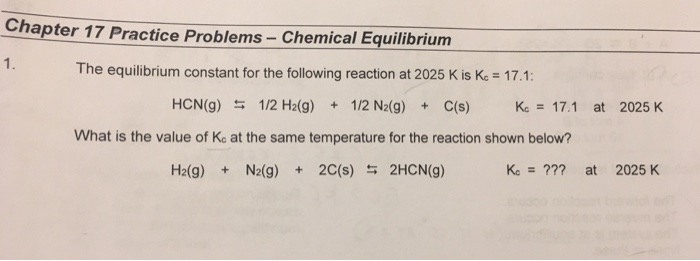
Solved Chapter 17 Practice Problems Chemical Equilibrium 1 Chegg Chemistry equilibrium practice problems covering equilibrium expressions, concentrations, and le chatelier's principle. We'll learn how to calculate equilibrium concentrations or the equilibrium constant (k) itself using stoichiometry and algebra; specifically, we'll practice how to set up an ice (initial,. In this study guide we will solve problems either to calculate the equilibrium constant or to determine the equilibrium concentrations of reactants and products once the system is at equilibrium. to calculate k c we would need at least one of the equilibrium concentrations. Calculate the equilibrium constant, keq, for the following reaction at 25 °c, if [no]eq= 0.106 m, [o2]eq= 0.122 m and [no2]eq= 0.129 m. ). Question: practice problems in equilibrium (set 1) part a: finding k a (g) 3 b (g) 2 c () 1. initially [a] 0.52 m and [b) 1.82 m. at equilibrium [a] 0.22 m. find k. 2. [c] 1.55 m, and (cheq 0.044 m. determine the value of k. 3. initially [c] 0.00455 m; at equilibrium [b] 0.00035. what is k? 2 os (g)3 02 (g) 4. [o3]. 0.5 m, and [ola 0.5 m. To describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single product and a single reactant, the conversion of n butane to isobutane (equation 15.26), for which k = 2.6 at 25°c.

Solved Practice Problems In Equilibrium Set 1 Part A Chegg In this study guide we will solve problems either to calculate the equilibrium constant or to determine the equilibrium concentrations of reactants and products once the system is at equilibrium. to calculate k c we would need at least one of the equilibrium concentrations. Calculate the equilibrium constant, keq, for the following reaction at 25 °c, if [no]eq= 0.106 m, [o2]eq= 0.122 m and [no2]eq= 0.129 m. ). Question: practice problems in equilibrium (set 1) part a: finding k a (g) 3 b (g) 2 c () 1. initially [a] 0.52 m and [b) 1.82 m. at equilibrium [a] 0.22 m. find k. 2. [c] 1.55 m, and (cheq 0.044 m. determine the value of k. 3. initially [c] 0.00455 m; at equilibrium [b] 0.00035. what is k? 2 os (g)3 02 (g) 4. [o3]. 0.5 m, and [ola 0.5 m. To describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single product and a single reactant, the conversion of n butane to isobutane (equation 15.26), for which k = 2.6 at 25°c.

Solved Practice Problems In Equilibrium Set 1 Part A Chegg Question: practice problems in equilibrium (set 1) part a: finding k a (g) 3 b (g) 2 c () 1. initially [a] 0.52 m and [b) 1.82 m. at equilibrium [a] 0.22 m. find k. 2. [c] 1.55 m, and (cheq 0.044 m. determine the value of k. 3. initially [c] 0.00455 m; at equilibrium [b] 0.00035. what is k? 2 os (g)3 02 (g) 4. [o3]. 0.5 m, and [ola 0.5 m. To describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single product and a single reactant, the conversion of n butane to isobutane (equation 15.26), for which k = 2.6 at 25°c.
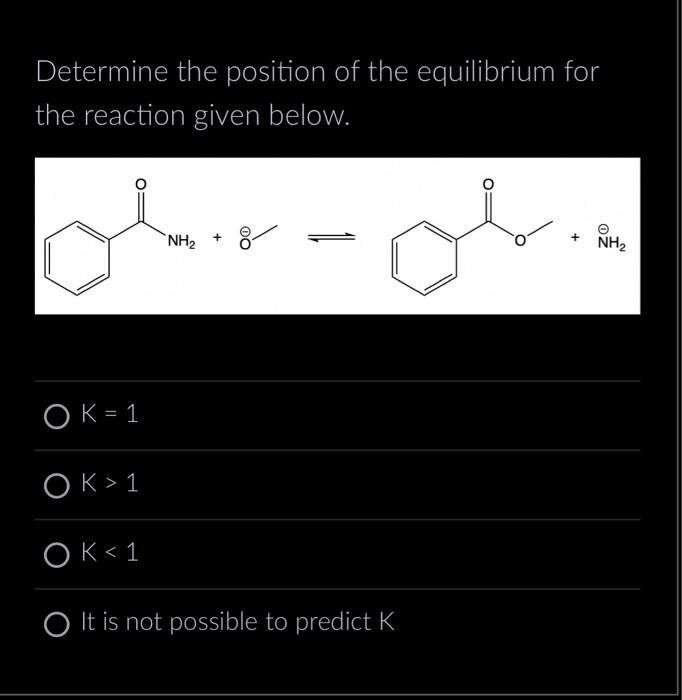
Solved Determine The Position Of The Equilibrium For The Chegg