
Solved Predict The Position Of Equilibrium For The Following Chegg Here’s the best way to solve it. ans (a)the equilibrium for the given reaction will be shifted to the … predict the position of equilibrium for the following reaction. not the question you’re looking for? post any question and get expert help quickly. For the following 10 questions use the following reaction system. uo2 (s) 4hf (g) > uf4 (g) 2h2o (g) suppose that this system has already reached equilibrium. predict the affect of each (1 10) of the following changes on the position of equilibrium. additional of uo2 is added to the system.

Solved Predict The Position Of Equilibrium For The Following Chegg The position of equilibrium in acid base reactions depends on the relative acidity of the reactants and products. phenol is a weaker acid compared to thiophenol, meaning thiophenol has a higher tendency to donate a proton (h ). For the following equilibrium reaction, predict the direction of the equilibrium shift by specifying if each stress will cause the concentration of reactants or products to increase. Answer the equilibrium position favors the reverse direction because sulfur is larger, making a the weaker base and c the stronger acid. To solve this problem, we need to apply le chatelier's principle, which helps us predict how a change in conditions will affect the position of equilibrium in a chemical reaction.
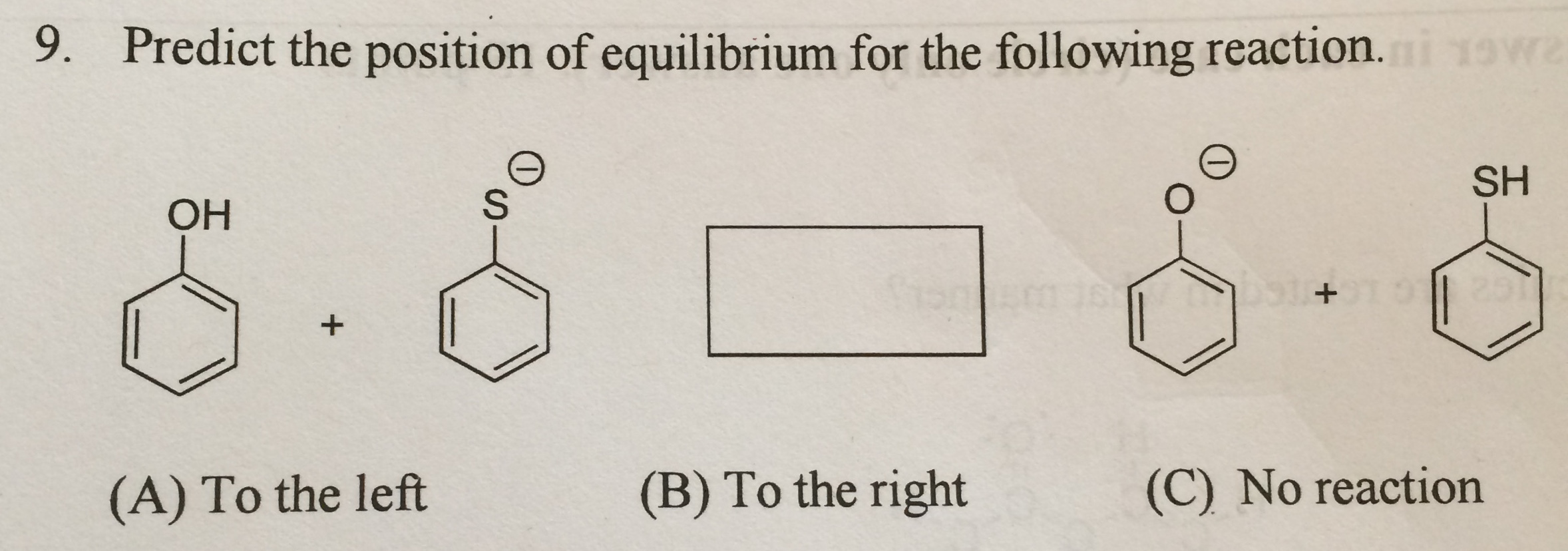
Solved Predict The Position Of Equilibrium For The Following Chegg Answer the equilibrium position favors the reverse direction because sulfur is larger, making a the weaker base and c the stronger acid. To solve this problem, we need to apply le chatelier's principle, which helps us predict how a change in conditions will affect the position of equilibrium in a chemical reaction. Question: when adding heat to the following reaction at its equilibrium position, in which direction the reaction will shift to?2so2 (g) o2 (g)§#8596;2so3 (g) heat no changeshifts to left (reverse)shifts to right (forward)cannot be predicted. Predict the position of the equilibrium for the reaction and provide a brief explanation. (the acidic protons are answered step by step solved by verified expert wilfrid laurier university. We'll learn how to calculate equilibrium concentrations or the equilibrium constant (k) itself using stoichiometry and algebra; specifically, we'll practice how to set up an ice (initial,. Step 1 the reaction in the image is an equilibrium between alkoxides and alcohols. to understand the direct.