
Sets Part 1 Pdf We discuss one electron ("atomic orbital") basis sets in quantum chemistry: slater type orbitals, gaussian type orbitals, and contracted gaussian type orbitals. Epr ii is a double zeta basis set with a single set of polarization functions and an enhanced s part: (6,1) [4,1] for h and (10,5,1) [6,2,1] for b to f. epr iii is a triple zeta basis set including diffuse functions, double d polarizations and a single set of f polarization functions.

Sets Part1 1 Pdf A basis set of the type st0 4 31g is one that could be used for a second row atom. the notation means that the core 1s electrons are described by expanding the slater type orbitals in four gaussians, whereas the valence 2s and 2p orbitals are expanded in three and one gaussians, respectively. Be aware that different basis sets are needed for hartree–fock and dft calculations on the one hand and electron correlation calculations (mpn, ci, cc) on the other. This discussion includes the topic of basis sets, which are a pre defined set of ao basis functions for each atom in the periodic table. instructor: prof. troy van voorhis. The most important basis sets are contracted sets of atom centered gaussian functions. the number of basis functions used depends on the number of core and valence atomic orbitals, and whether the atom is light (h or he) or heavy (everything else).

Basis Sets Sajeewa Pemasinghe This discussion includes the topic of basis sets, which are a pre defined set of ao basis functions for each atom in the periodic table. instructor: prof. troy van voorhis. The most important basis sets are contracted sets of atom centered gaussian functions. the number of basis functions used depends on the number of core and valence atomic orbitals, and whether the atom is light (h or he) or heavy (everything else). One of the three major decisions for the scientist is which basis set to use. there are two general categories of basis sets: basis sets were first developed by j.c. slater. slater fit linear least squares to data that could be easily calculated. the general expression for a basis function is given as:. Minimal basis set: “single zeta” (zeta commonly used for the exponent in sto ng) twice three times the basis functions for valence atomic orbitals: double zeta, triple zeta,. •basis sets are developed and optimized for individual atoms, and combined across atoms to form the orbitals for a molecule. –basis functions are atomic orbitals –separate into radial and spherical harmonic parts. atom a. in principle, the use of a basis set to represent a wavefunction is not an approximation. 3. The molecular orbitals are in general written as a linear expansion of a set of analytic functions, the basis set. basis sets are in general developed and optimized for individual atoms and the basis set for a molecule comprises then the basis sets of the atoms constituting the molecule.
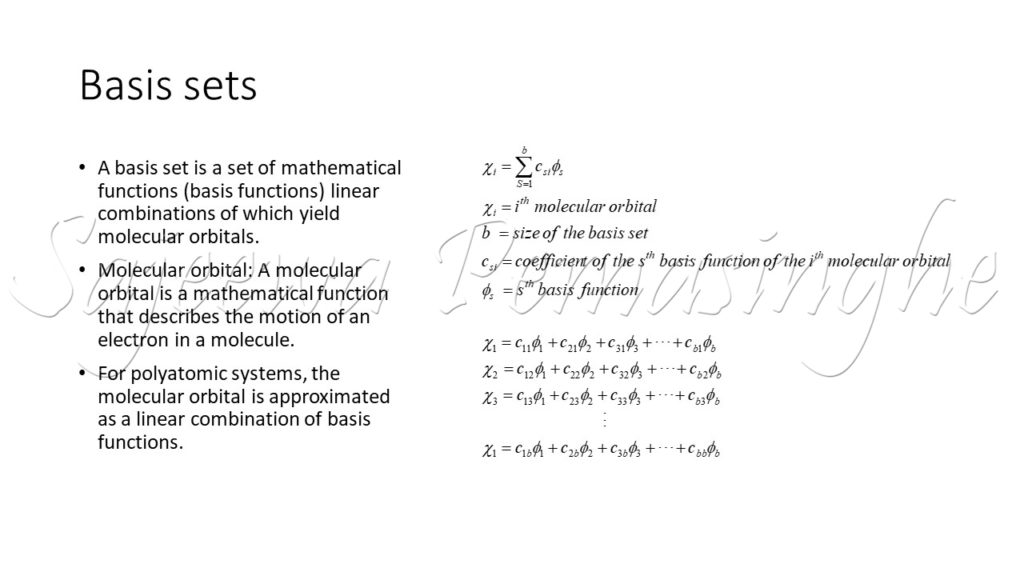
Basis Sets Sajeewa Pemasinghe One of the three major decisions for the scientist is which basis set to use. there are two general categories of basis sets: basis sets were first developed by j.c. slater. slater fit linear least squares to data that could be easily calculated. the general expression for a basis function is given as:. Minimal basis set: “single zeta” (zeta commonly used for the exponent in sto ng) twice three times the basis functions for valence atomic orbitals: double zeta, triple zeta,. •basis sets are developed and optimized for individual atoms, and combined across atoms to form the orbitals for a molecule. –basis functions are atomic orbitals –separate into radial and spherical harmonic parts. atom a. in principle, the use of a basis set to represent a wavefunction is not an approximation. 3. The molecular orbitals are in general written as a linear expansion of a set of analytic functions, the basis set. basis sets are in general developed and optimized for individual atoms and the basis set for a molecule comprises then the basis sets of the atoms constituting the molecule.

Basis Pdf •basis sets are developed and optimized for individual atoms, and combined across atoms to form the orbitals for a molecule. –basis functions are atomic orbitals –separate into radial and spherical harmonic parts. atom a. in principle, the use of a basis set to represent a wavefunction is not an approximation. 3. The molecular orbitals are in general written as a linear expansion of a set of analytic functions, the basis set. basis sets are in general developed and optimized for individual atoms and the basis set for a molecule comprises then the basis sets of the atoms constituting the molecule.

Basis Sets