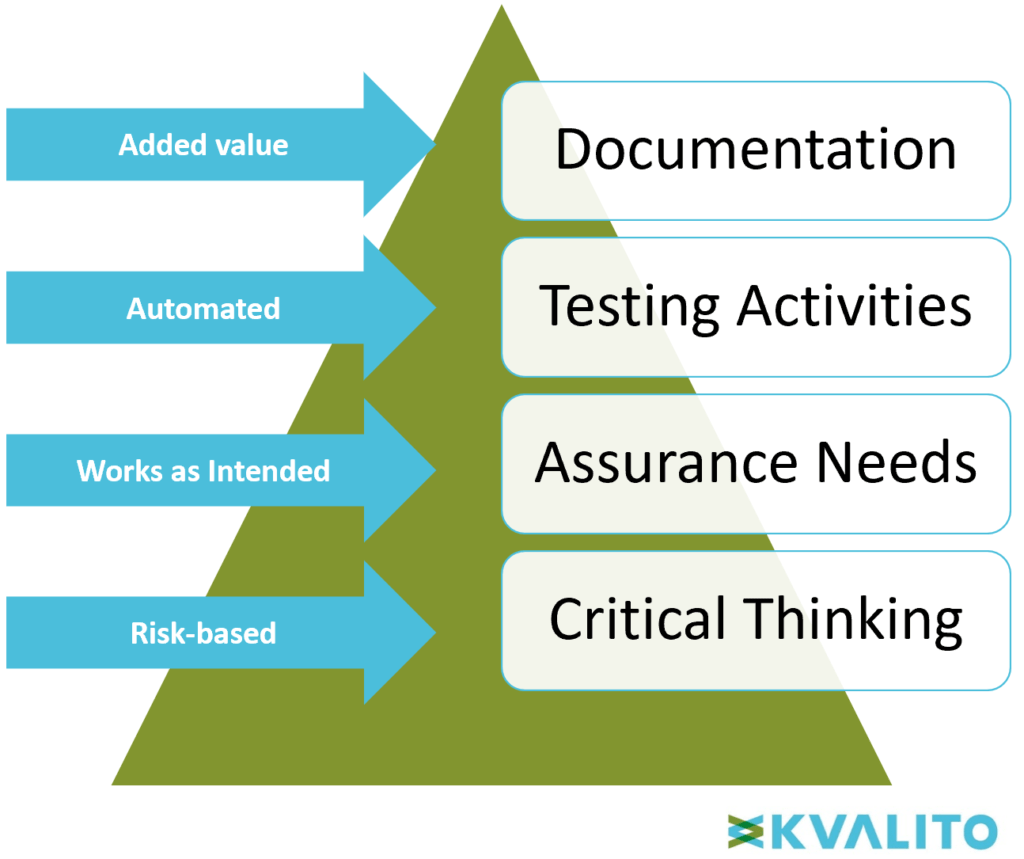
Why Computer System Validation Csv Engilifesciences In a risk based approach, critical thinking is used to prioritize the validation effort based on the level of risk associated with the computer system. these four aspects, together with the concepts outlined in ich q9, define a holistic approach to csv, and include the necessary added ingredient of critical thinking. A risk based approach to csv enables organizations to optimize validation efforts, improve compliance efficiency, and reduce unnecessary burdens without compromising regulatory adherence or patient safety.
Risk Based Computerized System Validation Csv And Computer Software The initiative found that the burden of computer systems validation (csv) deterred technology investments and as a result, inhibited quality best practice. as a result, the fda partnered with industry to strike a balance between promoting automation and value add csv activities. In the realm of computer system validation (csv), risk assessment stands as a cornerstone in ensuring the safety, efficacy, and compliance of software systems, particularly in regulated industries like pharmaceuticals and healthcare. the traditional risk assessment methodologies, while robust, have been evolving. A risk based approach in computerized systems validation (csv) enables better prioritization of validation activities by focusing efforts on systems that pose the highest risk to data integrity, product quality, patient safety, and regulatory compliance. If you wonder why needs a computer system validation, how the process looks like or you need some examples, have a look at this page. 1. introduction. 2. what do we need to validate? 2.1. what is validation? what is a computerized system? 2.2. how are they classified computerized systems? 2.3. what are the types of systems functionality and design?.
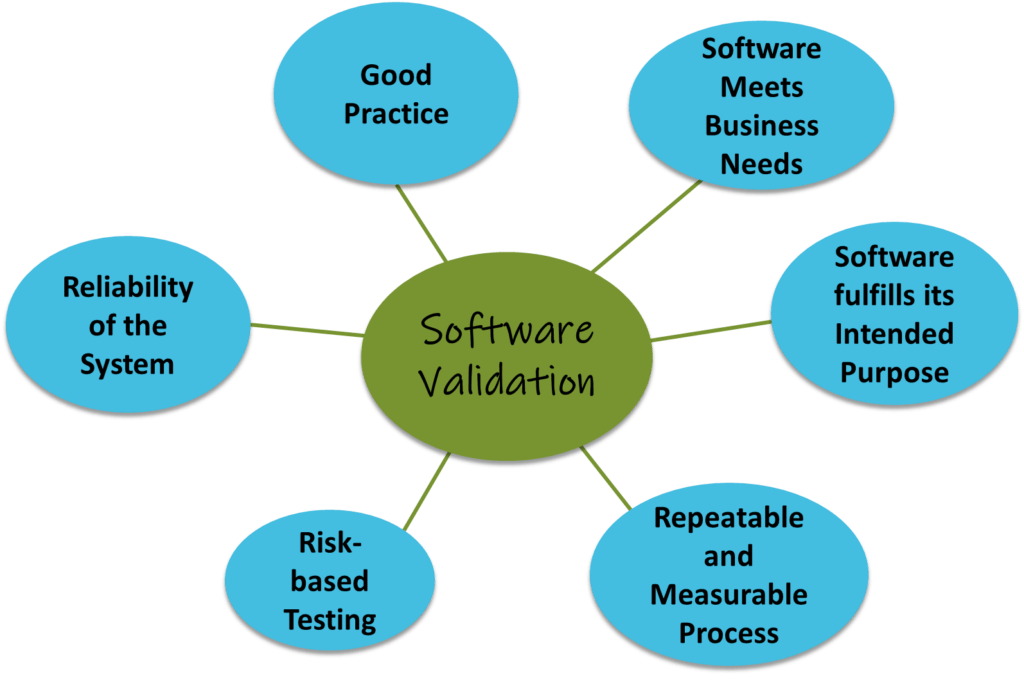
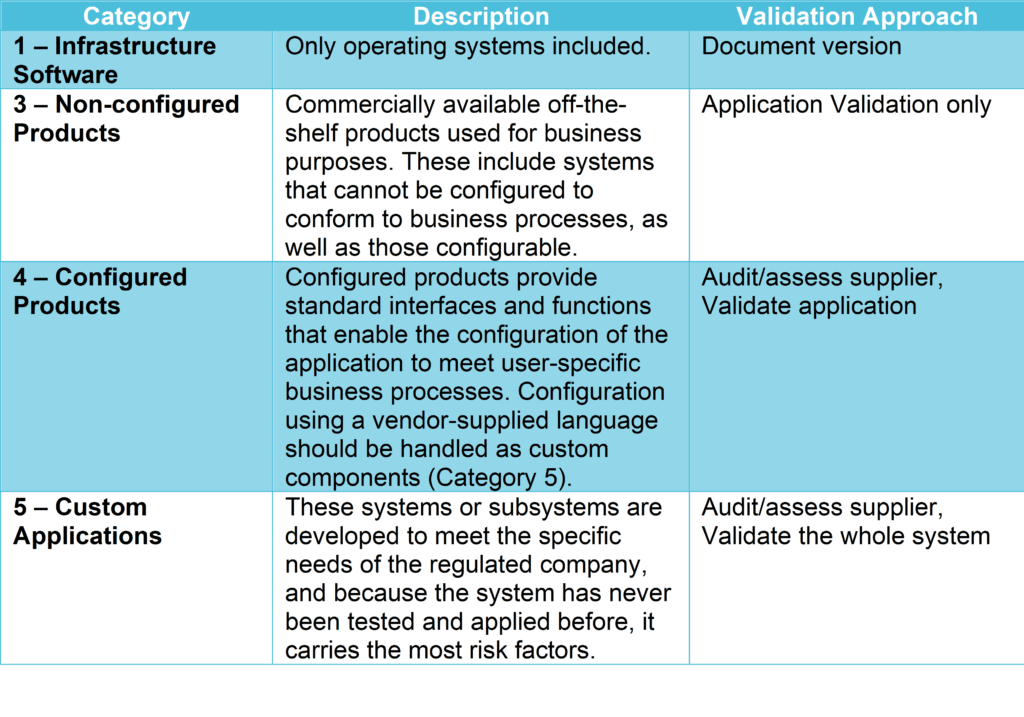
Risk Based Computerized System Validation Csv And Computer Software A risk based approach in computerized systems validation (csv) enables better prioritization of validation activities by focusing efforts on systems that pose the highest risk to data integrity, product quality, patient safety, and regulatory compliance. If you wonder why needs a computer system validation, how the process looks like or you need some examples, have a look at this page. 1. introduction. 2. what do we need to validate? 2.1. what is validation? what is a computerized system? 2.2. how are they classified computerized systems? 2.3. what are the types of systems functionality and design?. Computer system validation, or csv, is a modern, risk based methodology that confirms software used in pharmaceutical operations functions as intended. the risk based approach is based on critical thinking. Csv is not merely an industry recommendation; it's a regulatory necessity that bridges the gap between technology and regulatory compliance. this guide dives into csv's intricacies and sheds light on its best practices, as gleaned from a stimulating conversation with an industry expert, rashida ray. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does exactly what it is designed to do in a consistent and reproducible manner. the fda defines software validation as:. Risk based approach for computer system validation (csv) as mentioned before, iso 13485 requires the application of a risk based approach to determine the extent of the validation efforts towards a computer system.
Risk Based Computerized System Validation Csv And Computer Software Computer system validation, or csv, is a modern, risk based methodology that confirms software used in pharmaceutical operations functions as intended. the risk based approach is based on critical thinking. Csv is not merely an industry recommendation; it's a regulatory necessity that bridges the gap between technology and regulatory compliance. this guide dives into csv's intricacies and sheds light on its best practices, as gleaned from a stimulating conversation with an industry expert, rashida ray. Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does exactly what it is designed to do in a consistent and reproducible manner. the fda defines software validation as:. Risk based approach for computer system validation (csv) as mentioned before, iso 13485 requires the application of a risk based approach to determine the extent of the validation efforts towards a computer system.
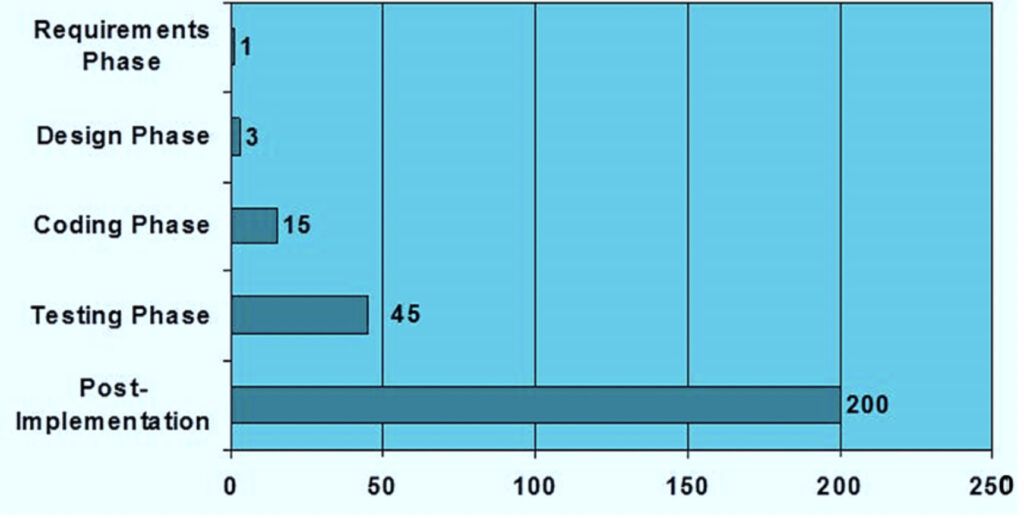
Risk Based Computerized System Validation Csv And Computer Software Computer systems validation (csv) is a documented process that is required by regulatory agencies around the world to verify that a computerized system does exactly what it is designed to do in a consistent and reproducible manner. the fda defines software validation as:. Risk based approach for computer system validation (csv) as mentioned before, iso 13485 requires the application of a risk based approach to determine the extent of the validation efforts towards a computer system.