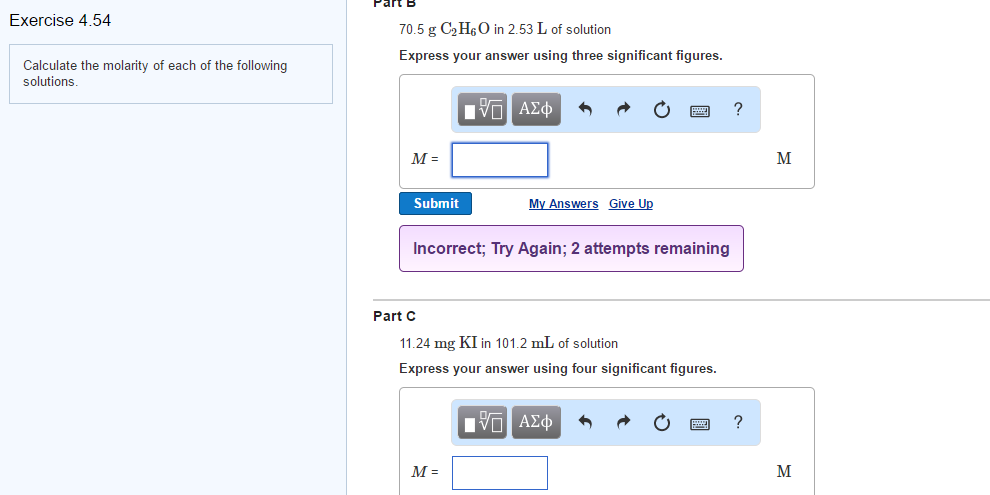
Solved Calculate The Molarity Of Each Of The Following Chegg There are 4 steps to solve this one. • molarity is defined as the number of moles of solute dissolved per litre of solution. molarity = moles of solute volume of solution in l . not the question you’re looking for? post any question and get expert help quickly. Problem 1: calculate the molarity of a solution containing 2 moles of nacl in 1 liter of water. solution. use the molarity formula: molarity (m) = (moles of solute) (liters of solution) => m = 2 mol 1 l = 2 mol l. problem 2: how many grams of kcl are needed to make 500 ml of a 1 m solution? (molar mass of kcl = 74.55 g mol) solution. 1.
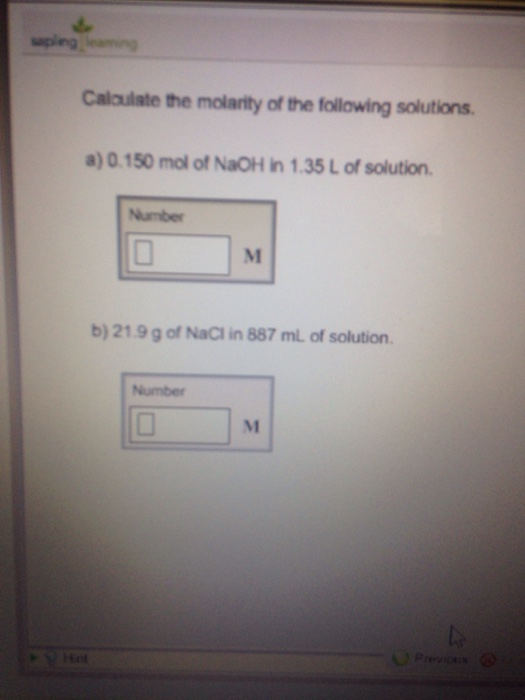
Solved Calculate The Molarity Of The Following Solutions Chegg Question 2 e) calculate each of the following concentration measurements: molarity (m), fraction (x), percentage by mass (% w w) and percentage by volume (% v v). ups 2011 2012: a solution is prepared by dissolving 16.0 g of calcium chloride, c ac l2 in 64.0 g of water. the solution formed has a density of 1.18 g ml−1. To find the molarity of the solution: suppose you need to prepare 2 liter of a 1 m solution of potassium nitrate (kno 3). the molecular weight of kno 3 is 101.1 g mol. how much potassium nitrate should you weigh out? first, rearrange the molarity equation to solve for the mass of solute m:. Calculate the molarity of a solution. as stated previously, chemists have defined several types of concentrations, which each use a different chemically acceptable unit, or combination of units, to indicate the amount of solute that is dissolved in a given amount of solvent. This calculator can solve problems on the molarity or molar concentration of a solute in a solution. first, it can calculate the molar concentration of a solute given a solute chemical formula, the mass of the solute and the volume of the solution.
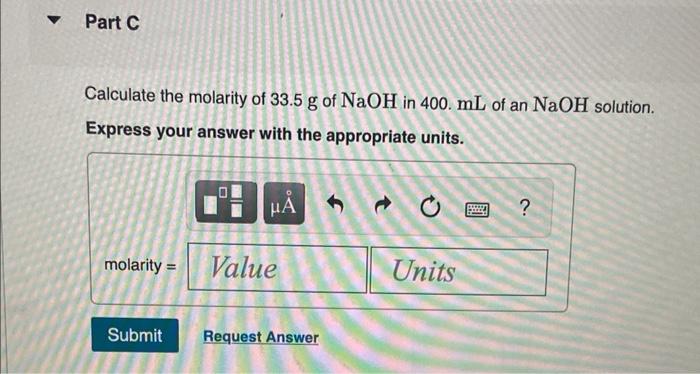
Solved Calculate The Molarity Of The Following Solutions Chegg Calculate the molarity of a solution. as stated previously, chemists have defined several types of concentrations, which each use a different chemically acceptable unit, or combination of units, to indicate the amount of solute that is dissolved in a given amount of solvent. This calculator can solve problems on the molarity or molar concentration of a solute in a solution. first, it can calculate the molar concentration of a solute given a solute chemical formula, the mass of the solute and the volume of the solution. Molarity is defined as the number of moles of solute in exactly 1 liter (1 l) of the solution: a 355 ml soft drink sample contains 0.133 mol of sucrose (table sugar). what is the molar concentration of sucrose in the beverage?. Use this calculator to determine the molar concentration (i.e., molarity) of a solution. all parameters of the equation can be calculated (solution concentration, solute mass, solution volume, and solute molecular weight). Boy, does it! the molarity of a solution is calculated by taking the moles of solute and dividing by the liters of solution. this is probably easiest to explain with examples. example #1: suppose we had 1.00 mole of sucrose (its mass is about 342.3 grams) and proceeded to mix it into some water. it would dissolve and make sugar water. Calculate the molarity of each of the following solutions: there is about 1.0 g of calcium, as ca 2 , in 1.0 l of milk. what is the molarity of ca 2 in milk? what volume of a 1.00 m fe (no 3) 3 solution can be diluted to prepare 1.00 l of a solution with a concentration of 0.250 m?.

Solved Calculate The Molarity Of The Following Solutions Chegg Molarity is defined as the number of moles of solute in exactly 1 liter (1 l) of the solution: a 355 ml soft drink sample contains 0.133 mol of sucrose (table sugar). what is the molar concentration of sucrose in the beverage?. Use this calculator to determine the molar concentration (i.e., molarity) of a solution. all parameters of the equation can be calculated (solution concentration, solute mass, solution volume, and solute molecular weight). Boy, does it! the molarity of a solution is calculated by taking the moles of solute and dividing by the liters of solution. this is probably easiest to explain with examples. example #1: suppose we had 1.00 mole of sucrose (its mass is about 342.3 grams) and proceeded to mix it into some water. it would dissolve and make sugar water. Calculate the molarity of each of the following solutions: there is about 1.0 g of calcium, as ca 2 , in 1.0 l of milk. what is the molarity of ca 2 in milk? what volume of a 1.00 m fe (no 3) 3 solution can be diluted to prepare 1.00 l of a solution with a concentration of 0.250 m?.

Solved Calculate The Molarity Of The Following Solutions Chegg Boy, does it! the molarity of a solution is calculated by taking the moles of solute and dividing by the liters of solution. this is probably easiest to explain with examples. example #1: suppose we had 1.00 mole of sucrose (its mass is about 342.3 grams) and proceeded to mix it into some water. it would dissolve and make sugar water. Calculate the molarity of each of the following solutions: there is about 1.0 g of calcium, as ca 2 , in 1.0 l of milk. what is the molarity of ca 2 in milk? what volume of a 1.00 m fe (no 3) 3 solution can be diluted to prepare 1.00 l of a solution with a concentration of 0.250 m?.
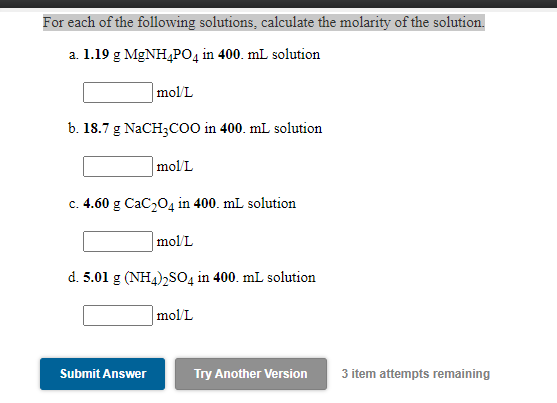
Solved For Each Of The Following Solutions Calculate The Chegg