
Differences Between Ionic And Covalent Compounds Pdf The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between atoms in a covalent bond. Learning about ionic and covalent bonds is an important part of any introductory chemistry course, and finding out the differences between bonds gives you an insight into why different materials behave and react in distinct ways.

Distinguishing Between Ionic And Covalent Compounds Lab Pdf Understanding the difference between ionic and covalent bonds is like learning the grammar of that language. ionic bonds represent clarity and structure, forged through strong opposites and governed by rules of charge and symmetry. covalent bonds represent nuance and subtlety, formed through sharing, shaped by geometry, and influenced by polarity. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an overall neutral ionic compound. The two main types of chemical bonds are ionic and covalent bonds. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the "sharing" is so unequal that an electron from atom a is completely lost to atom b, resulting in a pair of ions.
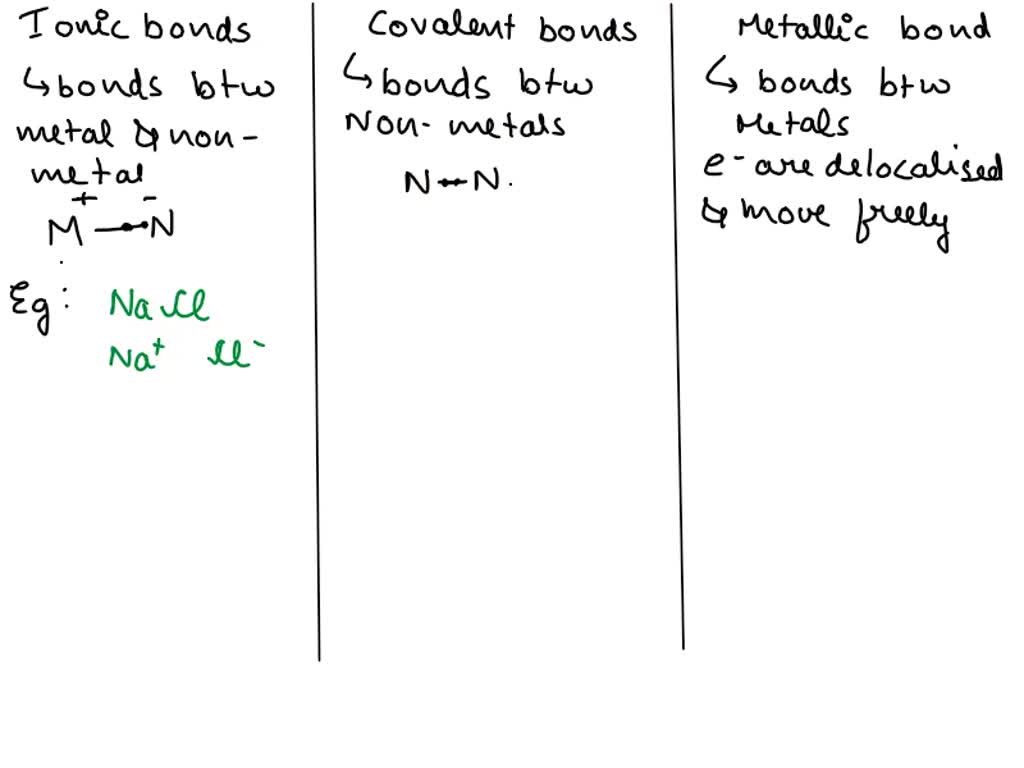
Solved Explain The Fundamental Differences Between Ionic Covalent And The two main types of chemical bonds are ionic and covalent bonds. an ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the "sharing" is so unequal that an electron from atom a is completely lost to atom b, resulting in a pair of ions. A covalent bond is a type of chemical bonding resulting from the mutual sharing of electrons between two atoms of the same or different elements. the bond is the electrostatic interaction between the electrons present in the orbit of one atom and the protons present in the nucleus of the other atom. An ionic bond is the electrostatic attraction between positively and negatively charged ions, while an ionic compound is a substance formed by the combination of atoms connected by ionic bonds, typically consisting of a metal and a nonmetal. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. the reaction components of covalent bonds are electrically neutral, whereas for ionic bonds they. Ionic bonds, with their electrifying exchange of electrons, create compounds that are soluble and conductive. in contrast, covalent bonds weave a world of shared electrons, giving rise to substances with incredible strength and stability.