
Solved Part 3 Density In This Part We Will Analyze Some Chegg You need to calculate volume and density ρ for both measurements. and then compare experimental ρruler and ρvernier with the actual values of denstiy of aluminium and steel ρaluminium = 2.7 g cm3 and ρsteel = 7.86 g cm3. To see all my chemistry videos, check out socratic.org chemistrywe'll practice solving density example problems. we'll look at how to use the density n.

Solved Part 3 Density In This Part We Will Analyze Some Chegg The density of a material at a given temp is constant > why we can use density as a way to help identify unknown substances density also determines whether an immiscible liquid will float sink in water. You will learn the technique for measuring the density of any liquid by experimentally determining the density of water then comparing it to the true value obtained from the crc handbook . you will need to pay close attention to the proper use of a graduated cylinder and a balance. Part 1: determining density using mathematical dimensions 1. tare the balance. 1. place the copper cylinder on the balance and record the mass of the copper cylinder. 2. using your ruler, measure the length and diameter of the cylinder. 3. record both measurements with units of centimeters in your data sheet. Given the data you collected for the volume of water displaced by 45 g of unknown metal, what is the density of the unknown metal? choose the value closest to your result.

Solved Part 3 Density In This Part We Will Analyze Some Chegg Part 1: determining density using mathematical dimensions 1. tare the balance. 1. place the copper cylinder on the balance and record the mass of the copper cylinder. 2. using your ruler, measure the length and diameter of the cylinder. 3. record both measurements with units of centimeters in your data sheet. Given the data you collected for the volume of water displaced by 45 g of unknown metal, what is the density of the unknown metal? choose the value closest to your result. Part iii determination of solution concentration using graphical trend line relationship between density and concentration the main goal of this part of the experiment was to find the density of five known concentrations which were 0%, 4%, 8%, 12%, and 16% of nacl solutions, plus another one with an unknown concentration. This document discusses density and provides examples of calculating density for various materials. it defines density as the ratio of an object's mass to its volume. The solution for the above question is provided in the space bellow. the olesect locm 9.0 cm height of the object 12 cm 12.cm volume o. Question: part 3: density in this part, we will analyze some data set to calculate the density of sphere and cylinder. sphere is made by steel, cylinder made by aluminium. measurements are taken by using a ruler and vernier caliper. you need to calculate volume and density for both measurements.
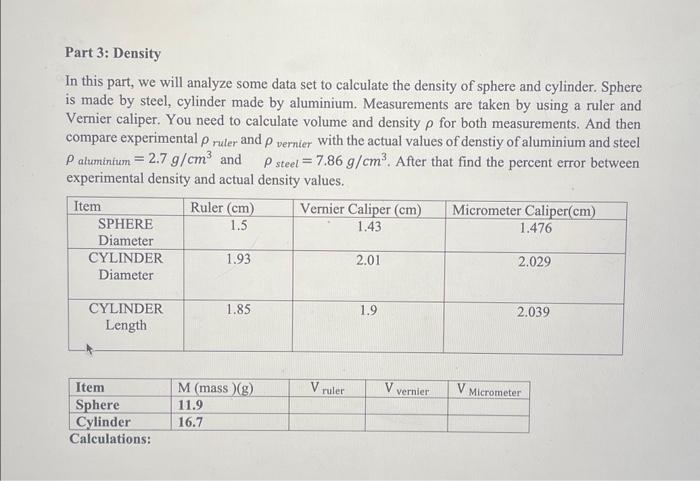
Solved Part 3 Density In This Part We Will Analyze Some Chegg Part iii determination of solution concentration using graphical trend line relationship between density and concentration the main goal of this part of the experiment was to find the density of five known concentrations which were 0%, 4%, 8%, 12%, and 16% of nacl solutions, plus another one with an unknown concentration. This document discusses density and provides examples of calculating density for various materials. it defines density as the ratio of an object's mass to its volume. The solution for the above question is provided in the space bellow. the olesect locm 9.0 cm height of the object 12 cm 12.cm volume o. Question: part 3: density in this part, we will analyze some data set to calculate the density of sphere and cylinder. sphere is made by steel, cylinder made by aluminium. measurements are taken by using a ruler and vernier caliper. you need to calculate volume and density for both measurements.

Solved Part 3 Density Problems Density Mass Volume For Chegg The solution for the above question is provided in the space bellow. the olesect locm 9.0 cm height of the object 12 cm 12.cm volume o. Question: part 3: density in this part, we will analyze some data set to calculate the density of sphere and cylinder. sphere is made by steel, cylinder made by aluminium. measurements are taken by using a ruler and vernier caliper. you need to calculate volume and density for both measurements.

Densities Of Solidsfor Part I And Part Iii Calculate Chegg