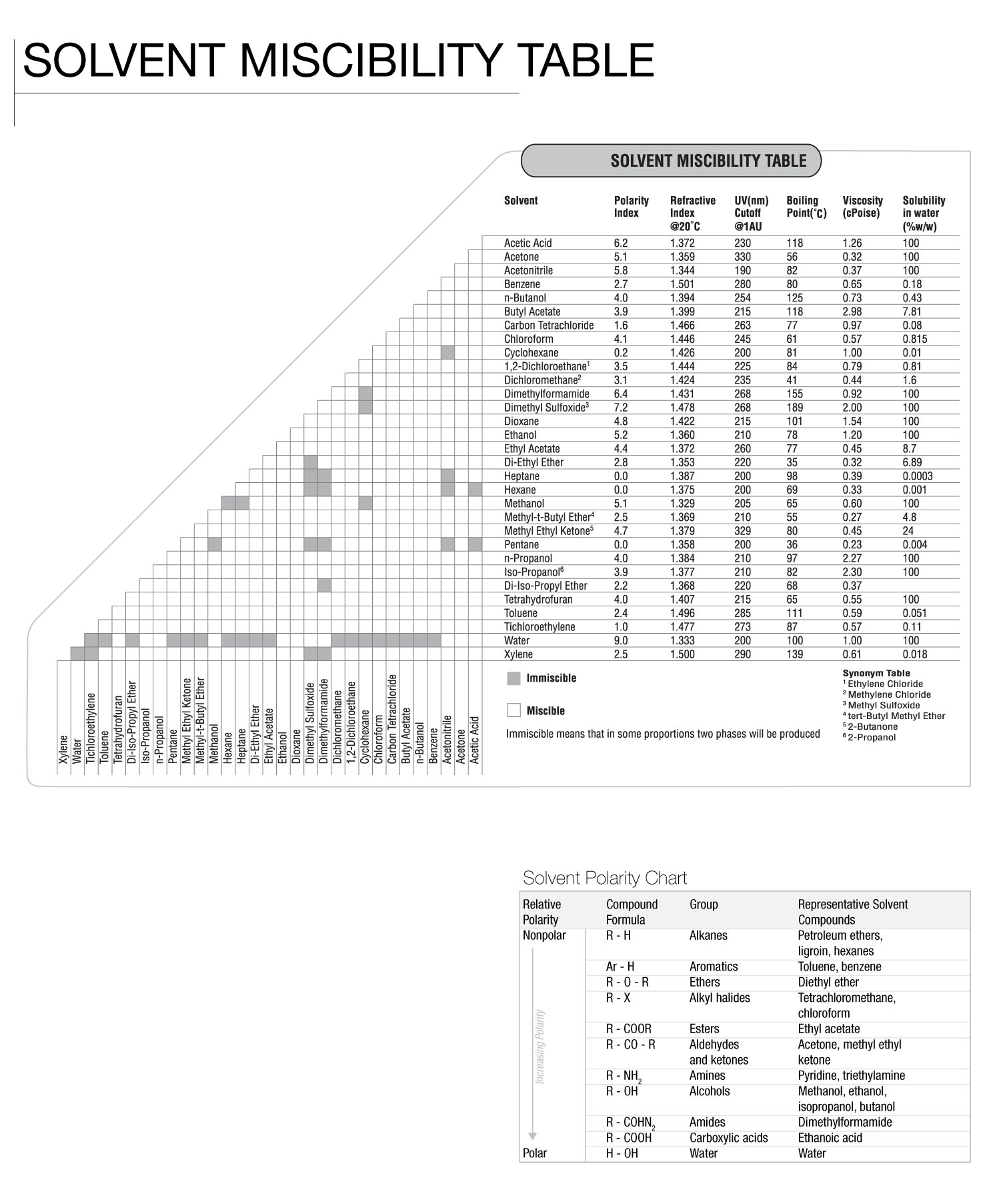
Solvent Miscibility Chart Analytical Future4200 A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. major uses of solvents are in paints, paint removers, inks, and dry cleaning. [2]. : a usually liquid substance capable of dissolving or dispersing one or more other substances. : something that provides a solution. : something that eliminates or attenuates something especially unwanted. adjective he couldn't stay solvent after losing his business. examples are automatically compiled from online sources to show current usage.

Solvent Miscibility Chart A Visual Reference Of Charts Chart Master The solvent is the substance that dissolves the solute and the component of a chemical solution present in the greatest amount. while most common solvent are liquids, a solvent can be a solid or gas. Solvent, substance, ordinarily a liquid, in which other materials dissolve to form a solution. polar solvents (e.g., water) favour formation of ion s; nonpolar ones (e.g., hydrocarbon s) do not. solvents may be predominantly acidic, predominantly basic, amphoteric (both), or aprotic (neither). A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. for example, in a saltwater mixture, water functions as the solvent, and salt is the solute. A solvent is the component of a solution that is present in the greatest amount. it is the substance in which the solute is dissolved. usually, a solvent is a liquid. however, it can be a gas, solid, or supercritical fluid. the amount of solvent required to dissolve a solute depends on temperature and the presence of other substances in a sample.
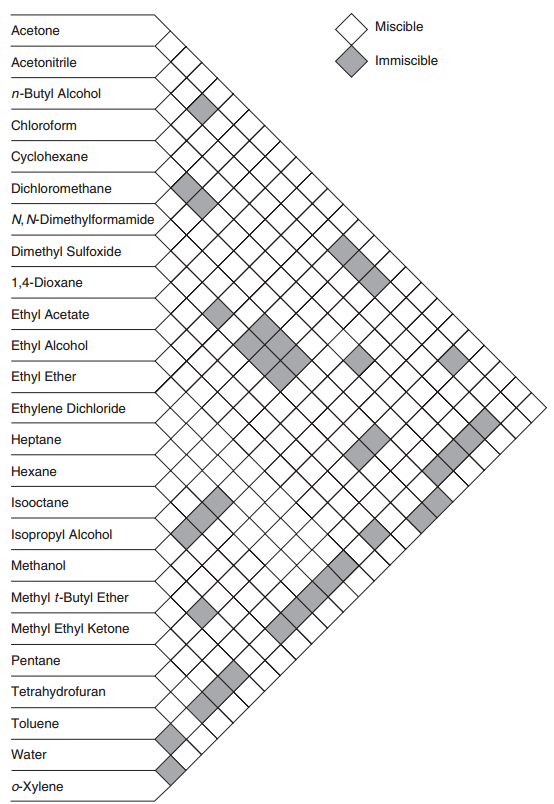
Solvent Miscibility Chart A Visual Reference Of Charts Chart Master A solvent is, by definition, any substance that will take other things (aka ‘solutes’) into solution. for example, in a saltwater mixture, water functions as the solvent, and salt is the solute. A solvent is the component of a solution that is present in the greatest amount. it is the substance in which the solute is dissolved. usually, a solvent is a liquid. however, it can be a gas, solid, or supercritical fluid. the amount of solvent required to dissolve a solute depends on temperature and the presence of other substances in a sample. A solvent is a substance that can dissolve an insoluble solute and create a solution. solvents are often liquids, although they can also be solids, gases, or supercritical fluids. When a solvent is added, the two reagents start to dissolve, and as they move around in the solution the two reagents encounter each other and start to react. at the beginning, you may not want to worry too much about the role of the solvent. A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. a solvent can be solid, liquid or gas. the molecules of the solvent work to put the solute molecules apart. Definition of solvent 1) a solvent is a substance, usually a liquid, that can dissolve other substances (called solutes) to form a solution. solvents are typically the major component of a solution and do not change their chemical structure during the dissolving process. some key points about solvents include:.
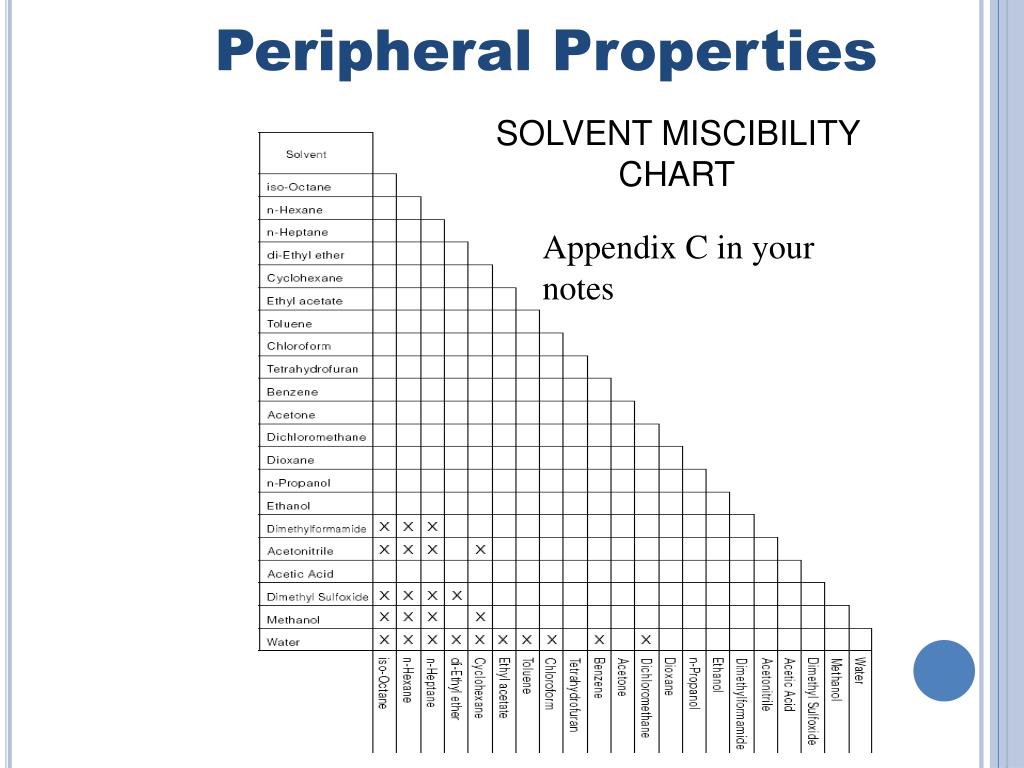
Solvent Miscibility Chart A Visual Reference Of Charts Chart Master A solvent is a substance that can dissolve an insoluble solute and create a solution. solvents are often liquids, although they can also be solids, gases, or supercritical fluids. When a solvent is added, the two reagents start to dissolve, and as they move around in the solution the two reagents encounter each other and start to react. at the beginning, you may not want to worry too much about the role of the solvent. A solvent is a molecule that has the ability to dissolve other molecules, known as solutes. a solvent can be solid, liquid or gas. the molecules of the solvent work to put the solute molecules apart. Definition of solvent 1) a solvent is a substance, usually a liquid, that can dissolve other substances (called solutes) to form a solution. solvents are typically the major component of a solution and do not change their chemical structure during the dissolving process. some key points about solvents include:.