
Molarity Calculations Educreations Calculate the molarity of a solution. as stated previously, chemists have defined several types of concentrations, which each use a different chemically acceptable unit, or combination of units, to indicate the amount of solute that is dissolved in a given amount of solvent. Molarity measures the concentration of a solution. it is defined as the quantity of a substance (solute) dissolved in a given volume of a liquid (solution). represented by the symbol m, it is widely used in physical chemistry, science, and everyday life. [1 4] the molarity of a solution is calculated using the following formula: [1 5].

Aim Doing Calculations Using The Molarity Formula Do This free molarity calculator determines any of the four variables in the molarity equation, given the other three. To calculate the molarity of a solution, divide the moles of solute by the liters of a solution. the answer is expressed in the unit mol l. know the basic formula for calculating molarity. molarity is equal to the number of moles of a solute divided by the volume of the solution in liters. In order to calculate molarity of a solution, follow the steps given below: step 1: calculate the moles of the solute present in the solution. step 2: mark the volume of solution in liters. step 3: use the formula for finding molarity of the solution' molarity = number of moles of solute volume of solution in liters. Molarity is calculated, using the formula above, by considering two components: volume and moles. in the case that moles of the compound are unknown, molar mass can be used to convert the compound from grams to moles. the periodic table provides the atomic masses used to calculate molar mass.
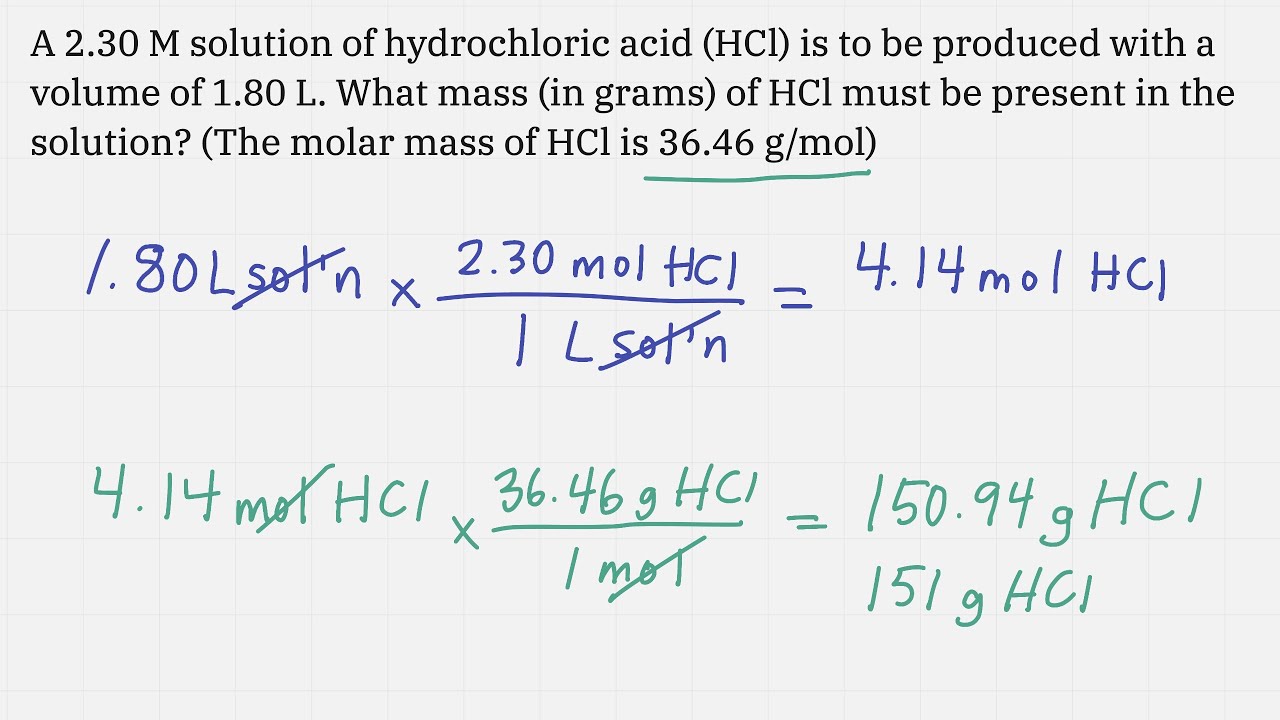
Using Molarity In Calculations Worksheet In order to calculate molarity of a solution, follow the steps given below: step 1: calculate the moles of the solute present in the solution. step 2: mark the volume of solution in liters. step 3: use the formula for finding molarity of the solution' molarity = number of moles of solute volume of solution in liters. Molarity is calculated, using the formula above, by considering two components: volume and moles. in the case that moles of the compound are unknown, molar mass can be used to convert the compound from grams to moles. the periodic table provides the atomic masses used to calculate molar mass. Molarity calculations. how to find molarity and use the molarity formula to solve for concentration. also, we'll do dilution problems and the dilution formula. Learn how to calculate the number of moles of a solute in a solution using molarity. a simple formula makes it easy to determine concentrations. Use this calculator to determine the molar concentration (i.e., molarity) of a solution. all parameters of the equation can be calculated (solution concentration, solute mass, solution volume, and solute molecular weight). Molarity measures how many moles of a solute is in one liter of a solution. convert the mass of the solute to moles using its molar mass, then find the solution volume in liters. divide the number of moles by liters of solution to find the molarity, like 0.20 m for kmno4.

Dilution Molarity And Volume Calculations A Chemistry Worksheet Molarity calculations. how to find molarity and use the molarity formula to solve for concentration. also, we'll do dilution problems and the dilution formula. Learn how to calculate the number of moles of a solute in a solution using molarity. a simple formula makes it easy to determine concentrations. Use this calculator to determine the molar concentration (i.e., molarity) of a solution. all parameters of the equation can be calculated (solution concentration, solute mass, solution volume, and solute molecular weight). Molarity measures how many moles of a solute is in one liter of a solution. convert the mass of the solute to moles using its molar mass, then find the solution volume in liters. divide the number of moles by liters of solution to find the molarity, like 0.20 m for kmno4.

Molarity Calculations How To Find Molarity Of A Solution Use this calculator to determine the molar concentration (i.e., molarity) of a solution. all parameters of the equation can be calculated (solution concentration, solute mass, solution volume, and solute molecular weight). Molarity measures how many moles of a solute is in one liter of a solution. convert the mass of the solute to moles using its molar mass, then find the solution volume in liters. divide the number of moles by liters of solution to find the molarity, like 0.20 m for kmno4.