
Calibration Qualification And Validation Pdf Verification And Validation is an act, process, or instance to support or collaborate something on a sound authoritative basis. verification is the act or process of establishing the truth or reality of something. qualification is an act or process to assure something complies with some condition, standard, or specific requirements. for example:. Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed, work as expected, and fit for the intended use. validation is the collection and assessment of data from process design to commercial phase, which establishes objective evidence that a process can consistently deliver a quality product.

Mazen Mahmoud On Linkedin Validation Verification Calibrtion The action of proving and documenting that any process, procedure or method actually and consistently leads to the expected results. it can better understand that the validation is a documented evidence to and done to prove the consistency of the expected results of any process, procedure or method. related: validation in pharmaceutical. Where calibration is used to find the underlying errors, verification is used to see whether or not the apparatus works as it is supposed to be working. in calibration, all the known uncertainties are considered while comparing the received values with a stated standard. This white paper explores the fundamental differences between calibration and validation, outlines best practices for each, and provides guidance on integrating both into a quality management system (qms) to ensure compliance and product integrity. In summary, calibration focuses on ensuring the accuracy of measuring instruments, validation ensures the reliability and accuracy of methods and systems, and qualification verifies the suitability and compliance of equipment, facilities, and systems used in the pharmaceutical industry.

Calibration And Validation Pdf This white paper explores the fundamental differences between calibration and validation, outlines best practices for each, and provides guidance on integrating both into a quality management system (qms) to ensure compliance and product integrity. In summary, calibration focuses on ensuring the accuracy of measuring instruments, validation ensures the reliability and accuracy of methods and systems, and qualification verifies the suitability and compliance of equipment, facilities, and systems used in the pharmaceutical industry. What is the difference between validation, qualification, and calibration? calibration ensures measurement accuracy, qualification verifies equipment readiness, and validation ensures process consistency. Calibration supports validation and qualification: accurate instruments are essential for reliable validation and qualification activities. qualification as a prerequisite for validation: equipment qualification ensures that systems are ready for process validation. In summary, calibration ensures the accuracy of an instrument or measurement system, verification checks that a product or system meets the intended design or performance criteria, and validation tests a product or system to ensure that it meets the user's needs and expectations.

Calibration Verification Setup Guide Software And Version 4 29 13 What is the difference between validation, qualification, and calibration? calibration ensures measurement accuracy, qualification verifies equipment readiness, and validation ensures process consistency. Calibration supports validation and qualification: accurate instruments are essential for reliable validation and qualification activities. qualification as a prerequisite for validation: equipment qualification ensures that systems are ready for process validation. In summary, calibration ensures the accuracy of an instrument or measurement system, verification checks that a product or system meets the intended design or performance criteria, and validation tests a product or system to ensure that it meets the user's needs and expectations.
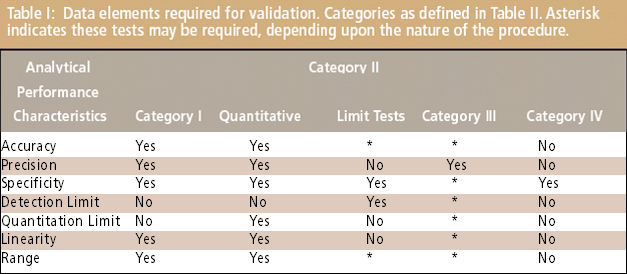
Validation Qualification Or Verification In summary, calibration ensures the accuracy of an instrument or measurement system, verification checks that a product or system meets the intended design or performance criteria, and validation tests a product or system to ensure that it meets the user's needs and expectations.